18++ Boiling point elevation freezing point depression worksheet answers Information
Home » Worksheets Online » 18++ Boiling point elevation freezing point depression worksheet answers InformationYour Boiling point elevation freezing point depression worksheet answers images are available. Boiling point elevation freezing point depression worksheet answers are a topic that is being searched for and liked by netizens now. You can Find and Download the Boiling point elevation freezing point depression worksheet answers files here. Find and Download all free photos.
If you’re looking for boiling point elevation freezing point depression worksheet answers pictures information connected with to the boiling point elevation freezing point depression worksheet answers interest, you have come to the right site. Our site always gives you suggestions for seeking the maximum quality video and picture content, please kindly hunt and locate more informative video articles and graphics that match your interests.
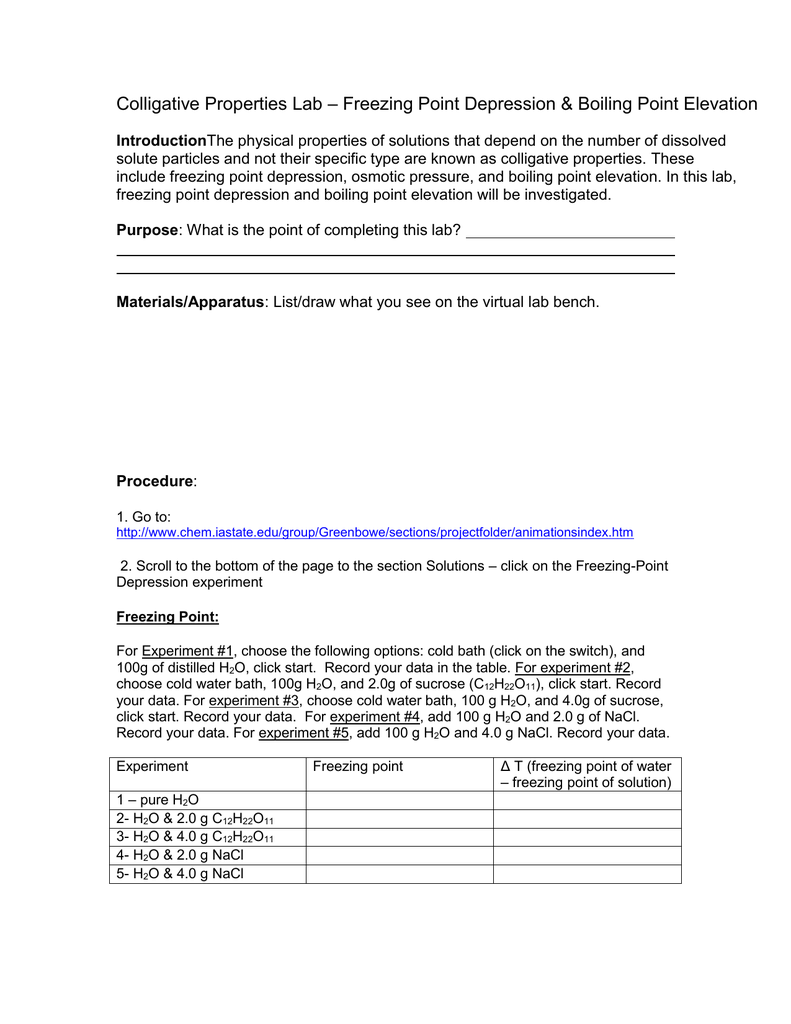
Boiling Point Elevation Freezing Point Depression Worksheet Answers. Change in the freezing point of the solution is T f 29103 C. The technique is insensitive and only useful for low molecular weight polymer eg M N less than 20000 gmol. We cannot express the concentration of the solution in molarity because it changes with temperature whereas molality is temperature independent. Colligative properties boiling point elevation worksheet Name.
 Freezing Point Depression Boiling From studylib.net
Freezing Point Depression Boiling From studylib.net
Discover learning games guided lessons and other interactive activities for children. Colligative properties boiling point elevation worksheet Name. Boiling-Point Elevation and Freezing-Point Depression. The amount of the elevation of the boiling point of a solution is given by Tb ikbm where kb is the molal boiling point elevation constant of the solvent and m is the molal concentration of the solute. Calculate the boiling point of the solution. K f freezing point depression constant water K f -186 o Cm Normal Boiling Point of Water 100.
Because the addition of a nonvolatile solute to a volatile solvent lowers the vapor pressure of the solution relative to the pure solvent the vapor pressure of the solution will be lower than the pure solvent at any temperature.
Discover learning games guided lessons and other interactive activities for children. The freezing points of solutions are all lower than that of the pure solvent and is directly proportional to the molality of the solute. Discover learning games guided lessons and other interactive activities for children. Calculate the freezing point of the solution. Ad Download over 20000 K-8 worksheets covering math reading social studies and more. Change in the freezing point of the solution is T f 29103 C.

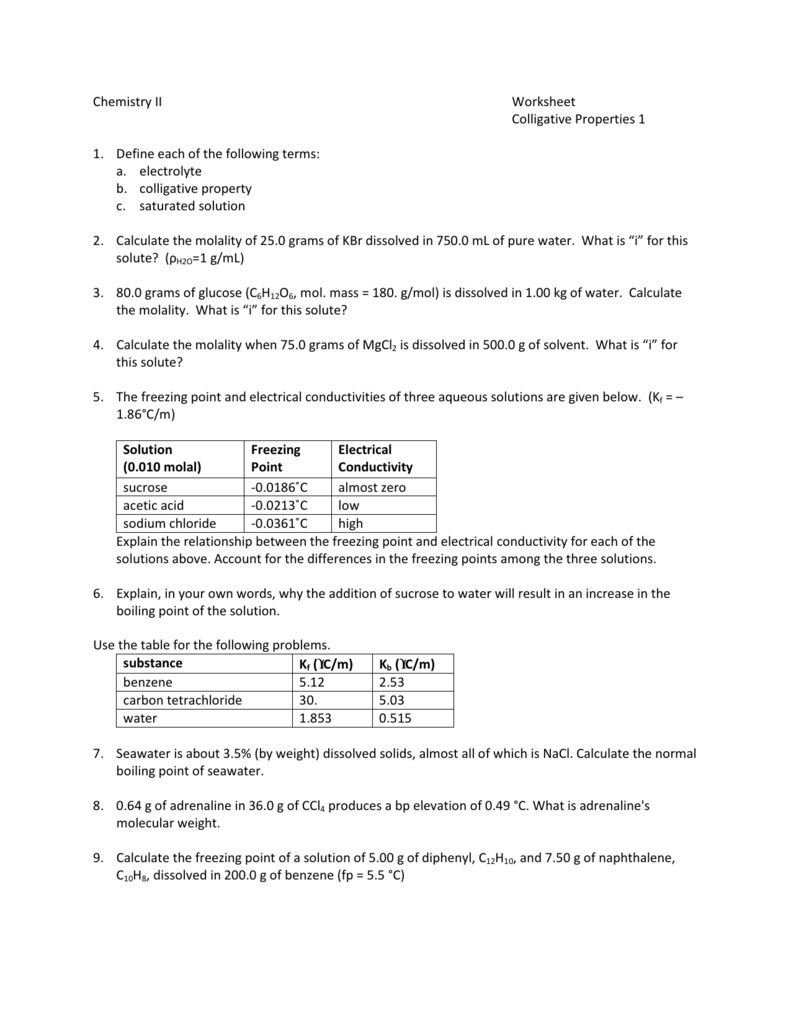
Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. Boiling Point ElevationFreezing Point Depression Worksheet Name. Colligative properties boiling point elevation worksheet Name. The freezing points of solutions are all lower than that of the pure solvent and is directly proportional to the molality of the solute. 54 Osmotic Pressure Another colligative property is osmotic pressure.
 Source: teacherspayteachers.com
Source: teacherspayteachers.com
Calculate the freezing point depression. Cyclohexane has a freezing point of 660 degree C and a K_f of 200 degree Cm. Boiling Point ElevationFreezing Point Depression Worksheet Name. 35B 36 Assume you have a 570m solution that raised the boiling point of the solution by 1620C. Molal freezing point depression constant Kf of the solution.
 Source: coursehero.com
Source: coursehero.com
Colligative properties boiling point elevation worksheet Name. Either vapor pressure boiling point elevation freezing point depression or most commonly osmotic pressure is measured and the appropriate equation is used to calculate the concentration molarity from. The freezing-point depression Delta T_f can be calculated in a similar manner Delta T_t K_f middot m in which m is the molality of the solution and K_f is the molal freezing-point-depression constant for the solvent. The amount of the elevation of the boiling point of a solution is given by Tb ikbm where kb is the molal boiling point elevation constant of the solvent and m is the molal concentration of the solute. Osmotic Pressure π i MRT.
 Source: studylib.net
Source: studylib.net
Like the boiling point elevation effect the freezing point depression effect is too small. 35B 36 Assume you have a 570m solution that raised the boiling point of the solution by 1620C. The amount of the depression of freezing point is given by Tf -ikfm where kf is the molal freezing point. Calculate the boiling point of the solution. FREEZING POINT DEPRESSION BOILING POINT PROPERTY ELEVATION m 92g 180 g mole0255 kg of solvent 20 m.
 Source: studylib.net
Source: studylib.net
Freezing Point Calculation Boiling Point Calculation. Change in the freezing point of the solution is T f 29103 C. Freezing point depression and boiling point elevation are examples of colligative properties. Discover learning games guided lessons and other interactive activities for children. 54 Osmotic Pressure Another colligative property is osmotic pressure.

I vant Hoff factor. I vant Hoff factor. Osmotic Pressure π i MRT. Discover learning games guided lessons and other interactive activities for children. Now The experimental method to determine the molecular mass of non-volatile solute by determining freezing points of pure solvent and solution of known concentration is called cryoscopy.
 Source: coursehero.com
Source: coursehero.com
Boiling Point Elevation ΔT i k b m solute. 35B 36 Assume you have a 570m solution that raised the boiling point of the solution by 1620C. Because the addition of a nonvolatile solute to a volatile solvent lowers the vapor pressure of the solution relative to the pure solvent the vapor pressure of the solution will be lower than the pure solvent at any temperature. Boiling-Point Elevation and Freezing-Point Depression. Molal freezing point depression constant Kf of the solution.

The freezing points of solutions are all lower than that of the pure solvent and is directly proportional to the molality of the solute. I vant Hoff factor. Camphor CloH160 has a molal freezing point depression. Boiling Point Elevation ΔT i k b m solute. K f freezing point depression constant water K f -186 o Cm Normal Boiling Point of Water 100.
 Source: coursehero.com
Source: coursehero.com
CHEMISTRY COLLIGATIVE PROPERTIES WORKSHEET m 152g 142 g mole0875 kg of solvent 122 m m. Colligative properties boiling point elevation worksheet Name. The amount of the elevation of the boiling point of a solution is given by Tb ikbm where kb is the molal boiling point elevation constant of the solvent and m is the molal concentration of the solute. Ad Download over 20000 K-8 worksheets covering math reading social studies and more. Discover learning games guided lessons and other interactive activities for children.
 Source: yumpu.com
Source: yumpu.com
Freezing point depression and boiling point elevation are examples of colligative properties. Freezing point depression and boiling point elevation are examples of colligative properties. Change in the freezing point of the solution is T f 29103 C. Either vapor pressure boiling point elevation freezing point depression or most commonly osmotic pressure is measured and the appropriate equation is used to calculate the concentration molarity from. 35B 36 Assume you have a 570m solution that raised the boiling point of the solution by 1620C.
 Source: lessonplanet.com
Source: lessonplanet.com
Normal Freezing Point of Water 000 o C. Now The experimental method to determine the molecular mass of non-volatile solute by determining freezing points of pure solvent and solution of known concentration is called cryoscopy. Boiling Point ElevationFreezing Point Depression Worksheet Name. _____ Student ID_____ Work in groups on these problems. Freezing Point Depression ΔT i k f m solute.
 Source: studylib.net
Source: studylib.net
Assume the K f of water is -186Cm. If you use 368mol of sucrose C 12 H 22 O 12 and dissolve this into 250kg of water what will be the change in the freezing point of your solution. Now The experimental method to determine the molecular mass of non-volatile solute by determining freezing points of pure solvent and solution of known concentration is called cryoscopy. Osmotic Pressure π i MRT. Colligative properties boiling point elevation worksheet Name.
 Source: coursehero.com
Source: coursehero.com
The freezing-point depression Delta T_f can be calculated in a similar manner Delta T_t K_f middot m in which m is the molality of the solution and K_f is the molal freezing-point-depression constant for the solvent. The freezing points of solutions are all lower than that of the pure solvent and is directly proportional to the molality of the solute. Discover learning games guided lessons and other interactive activities for children. Now The experimental method to determine the molecular mass of non-volatile solute by determining freezing points of pure solvent and solution of known concentration is called cryoscopy. Like the boiling point elevation effect the freezing point depression effect is too small.
 Source: studylib.net
Source: studylib.net
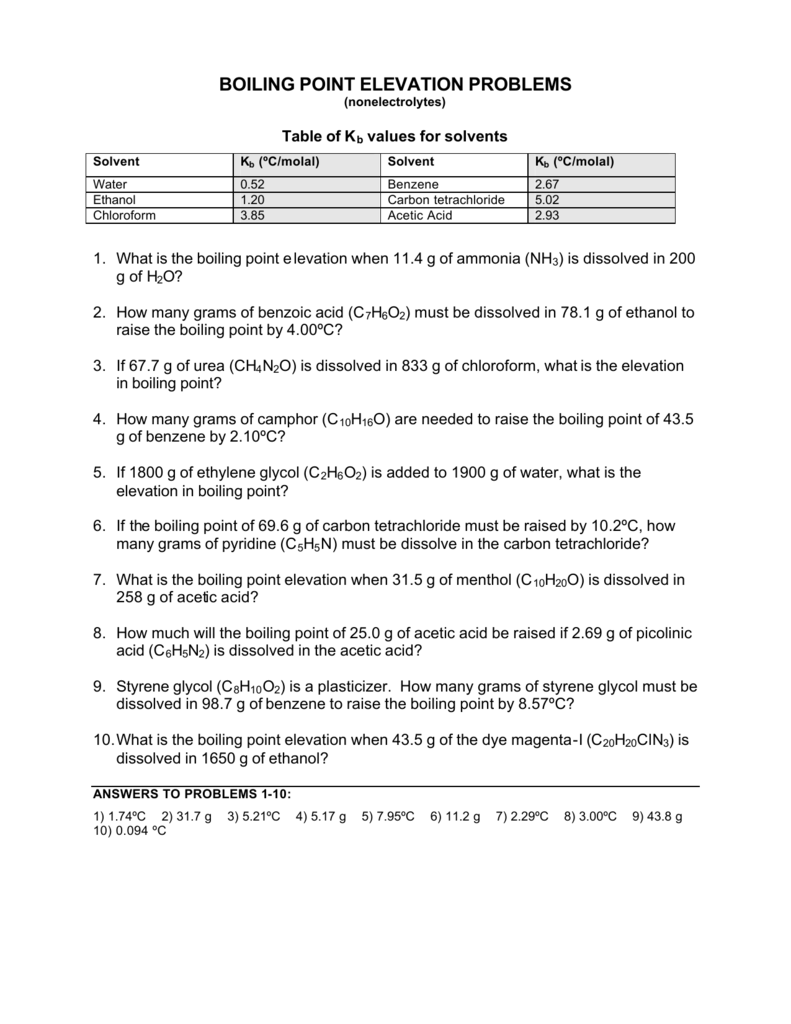
ΔT f -K f m K f 1855 from table above m 0262 m ΔT f -1855 0262 -0486 o C. The amount of the elevation of the boiling point of a solution is given by Tb ikbm where kb is the molal boiling point elevation constant of the solvent and m is the molal concentration of the solute. Freezing point depression and boiling point elevation are examples of colligative properties. Either vapor pressure boiling point elevation freezing point depression or most commonly osmotic pressure is measured and the appropriate equation is used to calculate the concentration molarity from. We found some Images about Freezing Point Depression And Boiling Point Elevation Worksheet Answer Key.
 Source: studylib.net
Source: studylib.net
Like the boiling point elevation effect the freezing point depression effect is too small. Raoult discovered that the addition of. 35B 36 Assume you have a 570m solution that raised the boiling point of the solution by 1620C. Boiling Point Elevation ΔT i k b m solute. Ad Download over 20000 K-8 worksheets covering math reading social studies and more.

The freezing points of solutions are all lower than that of the pure solvent and is directly proportional to the molality of the solute. Freezing Point Depression ΔT i k f m solute. Either vapor pressure boiling point elevation freezing point depression or most commonly osmotic pressure is measured and the appropriate equation is used to calculate the concentration molarity from. Freezing Point Calculation Boiling Point Calculation. FREEZING POINT DEPRESSION BOILING POINT PROPERTY ELEVATION m 92g 180 g mole0255 kg of solvent 20 m.
 Source: coursehero.com
Source: coursehero.com
What is the molal boiling point elevation constant KJ of the solution. Ad Download over 20000 K-8 worksheets covering math reading social studies and more. Assume the K f of water is -186Cm. Raoult discovered that the addition of. Calculate the freezing point of the solution.
 Source: studylib.net
Source: studylib.net
Looking at the formula for the boiling point elevation and freezing point depression we can see similarities between the two. ΔT f -K f m K f 1855 from table above m 0262 m ΔT f -1855 0262 -0486 o C. Camphor CloH160 has a molal freezing point depression. We found some Images about Freezing Point Depression And Boiling Point Elevation Worksheet Answer Key. Ad Download over 20000 K-8 worksheets covering math reading social studies and more.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point elevation freezing point depression worksheet answers by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.